The vapor pressure of water at 40.0ºC is 7.34 × 103 N/m2. Using the ideal gas law, calculate the density of water vapor in g/m3 that creates a partial pressure equal to this vapor pressure. The result should be the same as the saturation vapor density at that temperature 51.1 g/m3. Table 1 gives the vapor pressure of water at 20.0ºC as 2.33 × 103Pa Use the ideal gas law to calculate the density of water vapor in g/m3 that would create a partial pressure equal to this vapor pressure. Compare the result with the saturation vapor density given in the table. The ideal gas equation and the ideal gas constant, which express the ideal gas law provide a means for calculating air density for different pressures and temperatures.
Although this article is primarily about determining the density of air, the density of other gases at known temperature and gas pressure can also be estimated using the ideal gas law in the same way. On this slide you will find typical values of the properties of air at sea level static conditions for a standard day. We are all aware that pressure and temperature of the air depend on your location on the earth and the season of the year. And while it is hotter in some seasons than others, pressure and temperature change day to day, hour to hour, sometimes even minute to minute during severe weather.
The values presented on the slide are simply average values used by engineers to design machines. We also know that all of the state-of-the-gas variables will change with altitude, which is why the typical values are given at sea level, static conditions. Because the gravity of the Earth holds theatmosphere to the surface, as altitude increases, air density, pressure, and temperature decrease. The variation of the air from the standard can be very important since it affects flow parameters like the speed of sound. For a better understanding of how temperature and pressure influence air density, let's focus on a case of dry air. It contains mostly molecules of nitrogen and oxygen that are moving around at incredible speeds.
Use our particles velocity calculator to see how fast they can move! For example, the average speed of a nitrogen molecule with a mass of 14 u (u - unified atomic mass unit) at room temperature is about 670 m/s - two times faster than the speed of sound! Moreover, at higher temperatures, gas molecules further accelerate. As a result, they push harder against their surroundings, expanding the volume of the gas . And the higher the volume with the same amount of particles, the lower the density. Therefore, air's density decreases as the air is heated.
The temperature offset is the temperature deviation from the standard atmosphere 15 °C value. For example, if the actual air temperature near the Earth's surface is 25 °C then the offset will be 10 °C. The calculator allows the selection of various values of the Earth's radius used in calculations.
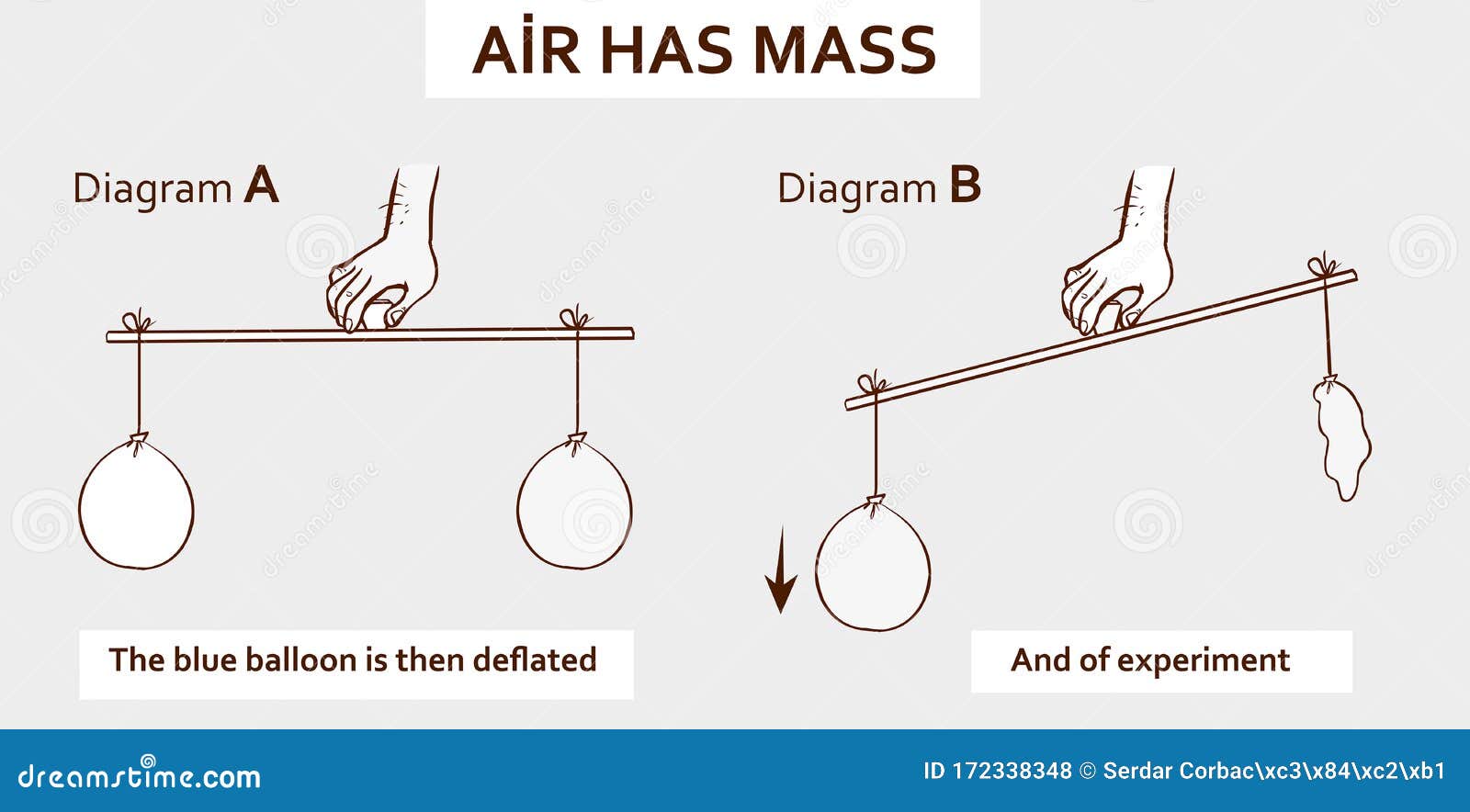
The capacity of air to hold water vapor is based on vapor pressure of water. The liquid and solid phases are continuously giving off vapor because some of the molecules have high enough speeds to enter the gas phase; see Figure 2a. If a lid is placed over the container, as in Figure 2b, evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance evaporation.
Then equilibrium has been achieved, and the vapor pressure is equal to the partial pressure of water in the container. Vapor pressure increases with temperature because molecular speeds are higher as temperature increases. Table 1 gives representative values of water vapor pressure over a range of temperatures. Air density equations Air contains a mixture of dry air and water vapor. The amount of water vapor is a function of the relative humidity; it is also related to the dew point temperature of the air. Because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point.
If the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. This vapor density and the partial pressure it creates are the saturation values. They increase with temperature and are independent of the presence of other gases, such as air.
Based on 21% oxygen and 79% nitrogen, the molecular weight of air is 28.8. At 20 C, the saturation vapor pressure of water is 2333 Pa. So the partial pressure of the water vapor in the air is 233 Pa. We do this using the IAPWS formula as shown below, but it should be noted that the much simpler formula of Davis10 will also be entirely adequate for this task at temperatures above 0 °C.
Air in human lungs has a temperature of 37.0ºC and a saturation vapor density of 44.0 g/m3. If 2.00 L of air is exhaled and very dry air inhaled, what is the maximum loss of water vapor by the person? Calculate the partial pressure of water vapor having this density, and compare it with the vapor pressure of 6.31 × 103 N/m2. The density of moist air is calculated as the sum of the density of the dry air component of the mixture plus the density of the saturated component of the mixture. In the first calculator, the vapour pressure of water vapour in saturated air at the nominated temperature is calculated and multiplied by the relative humidity to give the actual water vapour pressure.
The water vapour pressure is then subtracted from the total pressure to give the pressure of the dry component of the parcel. Densities of the two components are then calculated and summed to give the final answer. This is a good question to ask, because the air in a pipe friction loss, drag force, or pitot tube calculation, is indeed a real gas, not an ideal gas. Fortunately, however, many real gases behave almost exactly like an ideal gas over a wide range of temperatures and pressures. The ideal gas law doesn't work well for very low temperatures or very high gas pressures . For many practical, real situations, however, the ideal gas law gives quite accurate values for the density of air at different pressures and temperatures.
Where xv is the mole fraction of water vapor, f is the enhancement factor at the air temperature t and air pressure p, and td is the dew point . Equations and are standard equations for calculating mole fraction from dew point or relative humidity . Ciddor must also calculate mole fraction from partial pressure, and he appears to do this with equation , although his paper is slightly ambiguous on this point. An online air density calculator such as the one by Engineering Toolbox let you calculate theoretical values for air density at given temperatures and pressures.
The website also provides an air density table of values at different temperatures and pressures. These graphs show how density and specific weight decrease at higher values of temperature and pressure. If you need to calculate the density of dry air, you can apply the ideal gas law.
This law expresses density as a function of temperature and pressure. Like all gas laws, it is an approximation where real gases are concerned but is very good at low pressures and temperatures. Increasing temperature and pressure adds error to the calculation.
The density of air depends on many factors and can vary in different places. It mainly changes with temperature, relative humidity, pressure and hence with altitude . The air pressure can be related to the weight of the air over a given location. It is easy to imagine that the higher you stand, the less air is above you and the pressure is lower (check out our definition of pressure!). Therefore, air pressure decreases with increasing altitude.
In the following text, you will find out what is the air density at sea level and the standard air density. What you did is correct, although your calculated saturation vapor pressure of water is low by a factor of 100. Regarding what the other user said, dry air at the same total pressure as moist air has a higher density. If you redo you calculation for purely dry air, your calculation will confirm this. Here the difference between Edlén and Ciddor ranges from 1.4×10-7 at a wavelength of 1700 nm to 1.6×10-7 at 300 nm. These differences are probably well below anything of practical interest under such extreme conditions.
At 100 % humidity, the maximum difference within this range increases to 8×10-8, with the maximum error occurring at high humidity (100 % at 40 °C), high pressure , and short wavelength . This discrepancy arises from differences in the water vapor dispersion at the shortest wavelengths; for wavelengths longer than 400 nm, the maximum discrepancy over this range of atmospheric conditions is only 2.7×10-8. Equations and are not quit consistent with and ; they lead to different expressions for the fractional humidity that differ from each other by the ratio f/f. As a practical matter this factor is of negligible importance; the ratio is so close to unity that it affects the calculated index of refraction only at a level below 3×10-9 for temperatures below 50 °C.
The Edlén equation totally ignores the enhancement factor and other non-ideal gas effects relating to water vapor. In fact, the Edlén equation includes approximations for the water vapor term that are only accurate near 20 °C, and consequently the equation can be in error at high temperature and humidity. When temperatures approach 35 °C and high humidity is present, the Birch/Downs/Edlén equation will clearly not give as good results as does Ciddor. This result gives us good confidence in the two equations at the quoted level of uncertainty except under extreme conditions. Note that there have been no direct experimental measurements to support either equation under these environmental extremes.
Nevertheless, under some circumstances the Ciddor equation might be expected to give marginally better results, because it has been developed with broader applicability in mind and it arguably treats the dispersion more accurately. We calculate the saturation vapor pressure using the equations recommended by the International Association for the Properties of Water and Steam and described by Huang in reference 9. (See Huang's equations 4 an 8, which are also given here in Appendix A, Section A-I, Saturation Vapor Pressure.) By contrast, Ciddor1 and Bonsch and Potulski5 use formulae given by Davis10 and Giacomo8. At higher temperatures the differences in the calculated saturation vapor pressure are only slightly more substantial, increasing to 34 kPa at 100 °C, which would shift the index of refraction by roughly 1×10-8. We use the IAPWS formulation because it is generally accepted as the best formula available.
The estimated uncertainty of the IAPWS equation is 20 kPa at 100 °C but decreases rapidly to less than 2 kPa at 45 °C and 0.7 kPa at 20 °C. Where T is the kinetic temperature, which is the air temperature we usually measure using a thermometer. It is a function of the velocity of the molecules of gases of the Earth's atmosphere. M0 is the molecular mass of air at sea level and MH is the molecular mass of air at the altitude H.
At the altitudes below 100 km, molecular mass of air remains constant, therefore the molecular temperature is equal to the kinetic temperature. Where p is pressure in kPa, t is temperature in Celsius, and RH is relative humidity in percent . This formula is only valid for the standard, red He-Ne laser wavelength of approximately 633 nm, but this is not a serious limitation since 633 nm He-Ne lasers are used almost universally for displacement interferometry. The density of air is the mass per unit volume of atmospheric gases. The density of air, or how light it is, depends on the temperature and pressure of the air.
Typically, the value given for the density of air is at STP . The density of air is usually denoted by the Greek letter ρ, and it measures the mass of air per unit volume (e.g. g / m3). Dry air mostly consists of nitrogen (~78 %) and oxygen (~21 %).
The remaining 1 % contains many different gases, among others, argon, carbon dioxide, neon or helium. However, the air will cease to be dry air when water vapor appears. For any ideal gas, at a given temperature and pressure, the number of molecules is constant for a particular volume (see Avogadro's Law). So when water molecules are added to a given volume of air, the dry air molecules must decrease by the same number, to keep the pressure or temperature from increasing.
The calculator below can be used to calculate the air density and specific weight at given temperatures and atmospheric pressure. For temperatures below 0 °C, it is necessary to specify whether vapor pressure over ice [Huang's equation ] or over water [Huang's equation ] is to be calculated. The web page allows the user to enter either dew point (for vapor-water equilibrium) or frost point (for vapor-ice equilibrium).
When relative humidity is input, the calculation of psv assumes vapor-water equilibrium when the air temperature is above 0 °C and vapor-ice equilibrium below 0 °C. When we say humidity, we really mean relative humidity. Relative humidity tells us how much water vapor is in the air compared with the maximum possible. At its maximum, denoted as saturation, the relative humidity is 100%, and evaporation is inhibited. The amount of water vapor the air can hold depends on its temperature.
For example, relative humidity rises in the evening, as air temperature declines, sometimes reaching the dew point. At the dew point temperature, relative humidity is 100%, and fog may result from the condensation of water droplets if they are small enough to stay in suspension. Conversely, if you wish to dry something , it is more effective to blow hot air over it rather than cold air, because, among other things, hot air can hold more water vapor. Use this air density calculator to instantly find how tightly packed an object's molecules are, allowing you to estimate the ρ parameter basing on the local temperature and pressure conditions. This value is vital for many further calculations, such as determining the aerodynamic drag forces or the performance of wind turbines. Continue reading to get a better understanding of the relationship between the local weather and ρ, and learn what air density levels you can expect in various regions.
If we can treat air as an ideal gas, then the ideal gas law in this form can be used to calculate the density of air at different pressures and temperatures. Table 1 shows a comparison of the Ciddor and modified Edlén equations under various circumstances, calculated using this web site. The water vapor partial pressure or mole fraction was calculated using equations and in conjunction with the IAPWS vapor pressure formulae .
Humidity can be specified by entering relative humidity (0 % to 100 %), dew point, frost point , or partial pressure of water vapor. The web page will flag as suspect any humidity input that corresponds to a mole fraction of water vapor exceeding 20 % or corresponding to a relative humidity in excess of 85 % . A detailed discussion of humidity calculations follows below.
The discussion is included so as to provide detailed background information but it can be safely ignored by most users of this web page. Where (ntpf - ntp) is the water vapor correction, f is the partial pressure of water vapor, and σ is the wave number. This equation is written using the notation and symbols of Birch and Downs.
We use a different notation in the rest of this article. Rewritten in our notation, equation appears in Appendix A as equation . This modification of the Birch and Downs formula eliminates most of the discrepancy between the Birch and Downs formulation and the calculations of Ciddor at high temperature and humidity. Within the troposphere, the air temperature in Earth's atmosphere decreases with an increase in altitude. According to the international standard atmosphere and 1976 U.S. Standard Atmosphere , the rate of decrease of temperature is 6.5 K/km from sea level to 11 km or 36,089 feet.




















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.